Quality control of compounded medications
We have a responsibility to ensure patient safety and medication efficacy within our healthcare system. DrugLog™ is a cost-effective solution for verifying the identity and concentration of compounded injectable medications, quickly and efficiently. The quick, reliable and easy to use DrugLog™ device will verify the Right Drug and the Right Concentration in seconds and becomes an invaluable part of your Quality Control process.
Reduce medication errors
DrugLog™ is a robust stand-alone solution for reducing compounding errors. It verifies the identity and concentration of compounded injectables before they are administered to a patient. Whether DrugLog™ is used in the Pharmacy or at the Patient ward, you have peace of mind the patient is receiving what was intended.
Extra precaution, greater safety
DrugLog™ is an excellent complement to the already high safety standards of pharmaceutical compounding. Most cytotoxic drugs used in cancer treatment are individually prepared at the hospital pharmacy. Even in the hands of the most experienced professional, the risk of error is always present. Medical compounding demands extra precautions are taken during the entire process. DrugLog™ takes the guess work away during the Quality Control process and keeps you compliant.
Key features of DrugLog™
Reliable drug verification in seconds
DrugLog™ is a unique combination of cutting-edge software and reliable well-established hardware for absorption spectroscopy. Within a few seconds, users can determine whether a diluted or a compounded item is the Right Drug and the Right Concentration.
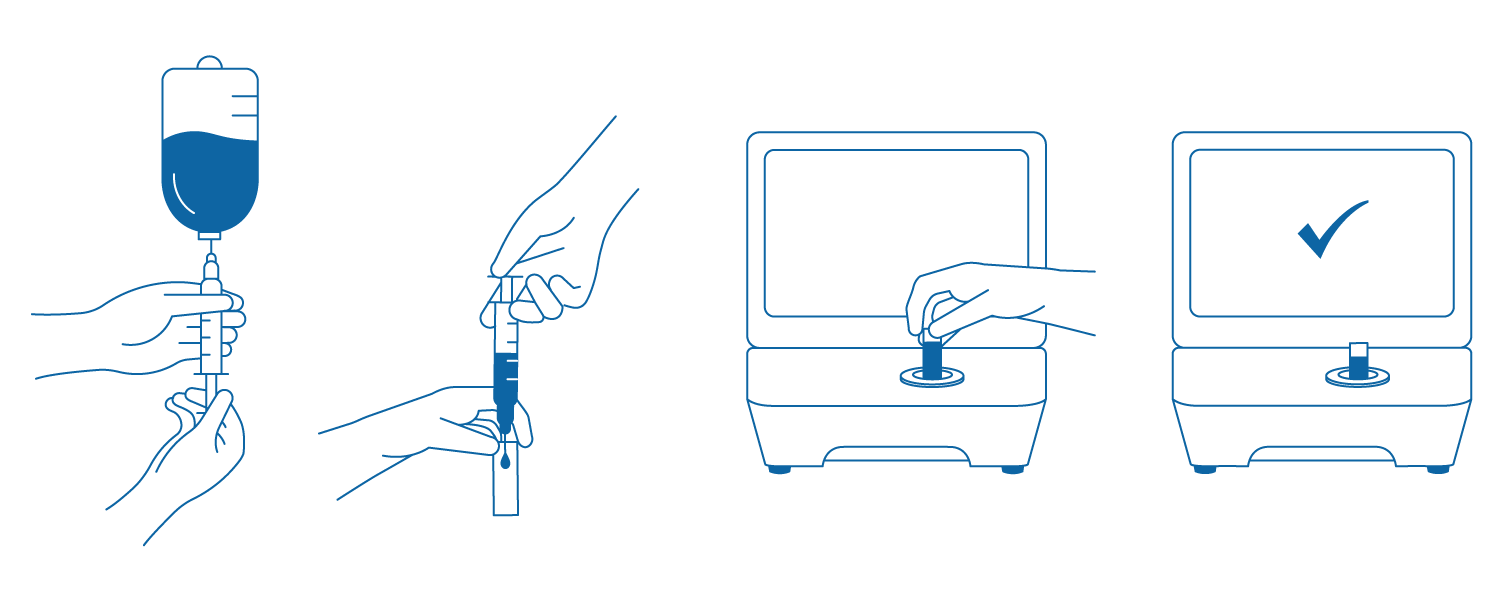
How it works
1. Withdraw a small sample (0.3–0.5 ml) from the prepared solution.
2. Inject the sample solution into the cuvette.
3. Insert the cuvette into the DrugLog™ unit.
4. Initiate the test and view the result within 2–3 seconds.
DrugLog™ is flexible for various compounding workflows
Whether you spot check your batch fills, or for independent verification of your compounding robot post maintenance or software upgrade, DrugLog™ gives you the peace of mind your compounding robot is operating as expected. DrugLog™ provides consistency in manual compounding processes and is an excellent tool for personnel evaluations. DrugLog™ is ideal for verifying your outsourced compounds when received.
Access data for review and analysis
Pharmacolog Dashboard™ provides remote access to a comprehensive overview of all pharmaceutical validations. Among endless benefits a senior pharmacist can for instance remotely assist colleagues with complicated compounding processes in the clean room, without being on site in the pharmacy.
Why DrugLog™?
Documentation

DrugLog™
Brochure
Download PDF

Pharmacolog Dashboard™
Brochure
Download PDF

Pharmacolog
Service & Support
Brochure
Download PDF

Customer Story -
Cliniques Universitaires Saint-Luc, Brussels, Belgium
Download PDF
