As someone who works in a healthcare facility, drug diversion puts everyone at risk. The behavior of individuals in your organization could lead to lawsuits, heavy fines, lost revenue and negative publicity. However, by far the most serious risk is that your patients may not be receiving the medication that they require or worse, end up with a bacterial infection. Inaction is not an option as it is the duty of all practitioners and healthcare facilities to be vigilant and take all the steps necessary to deter drug diversion or detect it when it occurs. The first step is to look for potential blind spots in your workflow. For a well-defined set of best practices to help you, please refer to the American Society of Health-System Pharmacists (ASHP) guidelines on preventing drug diversion.
Prevention is better than cure
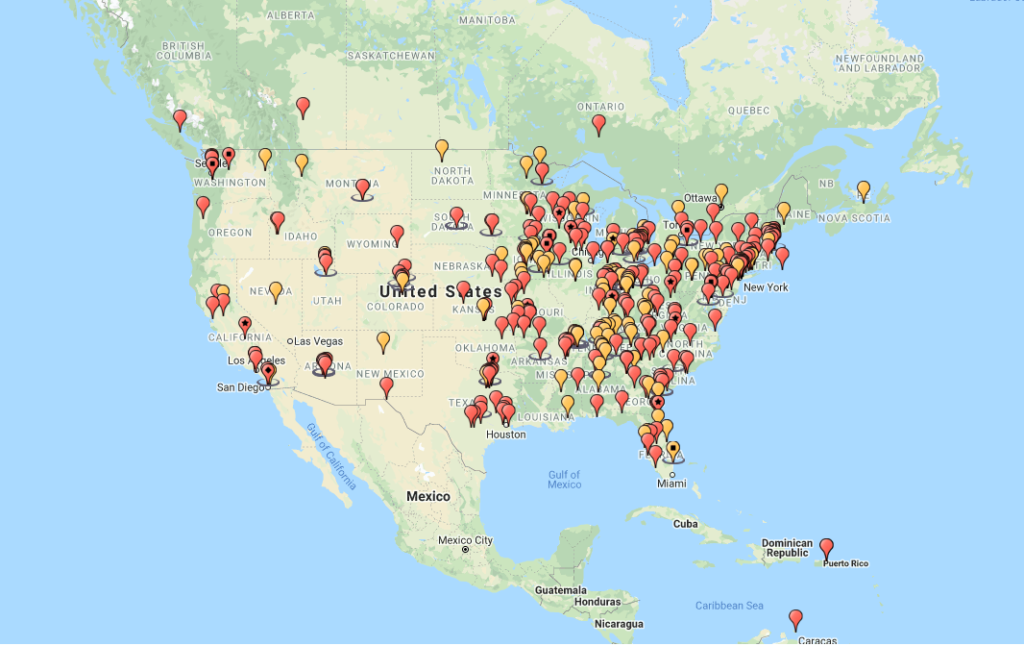
A cursory glance at the incident map provided by HealthCareDiversion.org paints a grim picture of a pervasive problem in the US. The map, which only shows diversions reported on the website, not all diversions in the US, gives details of incidents ranging in scale and severity. What is clear from the map, is that the more severe incidents have been allowed to go unnoticed for too long, many of them involving multiple staff members. Performing a gap analysis for your facility can help uncover weak points where drugs could potentially be diverted. Monitoring for drug diversion is a critical activity and a comprehensive monitoring program is crucial to preventing and detecting diversion. Just as the role of a security camera is to first act as a deterrent, an effective, and highly visible diversion monitoring program will significantly reduce the risk of employees diverting drugs since there is a higher risk of getting caught.
Gap Analysis
A gap analysis requires a thorough review of every touch point that your facility has with controlled substances. These touchpoints include:
- Documentation
- Storage and security
- Procurement
- Preparation and dispensing
- Prescribing
- Administration
- Waste and Return
- Overall monitoring
All of these touchpoints are critical in developing a comprehensive drug diversion prevention program, but I will focus on what Pharmacolog knows best, which is waste handling. Waste handling is a pivotal process and a key risk point for diversion. For additional guidance and guidelines on the other touchpoints, please refer to the American Society of Health-System Pharmacists guidelines on preventing drug diversion.
Waste handling
ASHP has identified waste handling as one of the five risk points for diversion. It is important that all associates are aware of the federal and state laws on waste handling of controlled substances as well as the penalties and fines associated with drug diversions. Waste can include products expiring, products prepared but not administered, overfill from a vial after dose is removed and product remaining from a partial dose removed from the original package. Controlled Substances should be wasted immediately using the approved method as defined by federal and state laws or regulations.
3 layers of protection
With waste handling, there are three layers of protection that can help detect or prevent diversion from occurring. They are, documentation, witnessing and analysis. This is exemplified nicely in the Minnesota Hospital Association Road Map where they say that wasting of all controlled substances requires an independent licensed witness and must be documented in the automated distribution machine. The only exception is when unusable product is returned to the pharmacy for assay.
Two of the layers are required by law, but ASHP guidelines indicate all three are necessary to help prevent and detect diversion.
Documentation
There are stringent requirements for documentation around controlled substances. During the products lifecycle, chain of custody and disposal of the product must be documented. Documentation include Automated Dispensing cabinets, State Controlled Drug Registers, DEA order and chain of custody forms. Reconciliations should be conducted and any discrepancies in documentation investigated as soon as possible.
This layer of protection is eroded if documentation is not taken seriously or kept up to date. This can cause errors to creep into your documents which have nothing to do with drug diversion, making it hard to determine if its diversion or error. Unfortunately, in most cases of drug diversion, document forgery has occurred and gone undetected. While documentation is required and critical, it cannot be relied upon alone.
Witnessing
Wasting of controlled substances should be witnessed by an independent, licensed individual. If performed according to the laws and regulations, it should reduce the possibility of drug diversion. However, as we know, providers sometimes take shortcuts despite this being a legal requirement and drug diversion still occurs.
There are several reasons why diversion still occurs with a witness. One is the work environment may prohibit effective witnessing procedures. When staff are overworked, the wasting procedures are difficult to comply with and witnesses simply enter their credentials into the ADM or initials the drug register. This practice also occurs when staff members are comfortable with each other and trust that the person they are witnessing is wasting the correct substance and volume.
Another way that diversion of waste can still occur with a witness is substitution. There are many ways in which controlled substances can be switched out or diluted prior to wasting which would go completely undetected by even the most experienced health care practitioner. There are also incidents of collusion between two practitioners AKA “the Buddy System”. In cases of collusion, you will notice that the same two staff members always witnessing each other even though there are other staff members around.
An effective drug diversion prevention program makes diversion difficult for the practitioner and easy for you to detect. The first two layers are extremely important, and in most cases effective, but they have their gaps. Incorporating the third layer of analysis helps fill in those gaps.
Analysis
One of the most common techniques for drug diversion is to dilute or replace the controlled substances with water or saline, while keeping the rest. A diluted sample would look completely normal on the documentation and a witness would not be able to tell. The only way to know for sure that the waste is the correct substance and concentration is to analyze it. Most documentation recommends that this is done randomly, especially in high volume use areas such as the OR, with spot testing in other areas of the facility. This provides an extra layer of difficulty for anyone looking to divert and increases the risk being caught during a random analysis.
Additional benefits to analyzing waste include medication error detection, procedure inefficiencies with practitioners, and performance improvement tool for technicians.


